Redox-Active Rotaxanes and Catenanes for Anion Sensing (DPhil, 2007-2011)
My doctoral research primarily involved the design and synthesis of solution-based interlocked molecules that could act as selective electrochemical anion sensors. This was achieved by appending and incorporating ferrocene into the structures of chloride templated rotaxanes and catenanes. These species bound chloride anions more strongly than monoanionic oxoanions in competitive solvent media, with a characteristic response for chloride also being observed electrochemically.

Key References:
Chemical Science, 2012, 3, 1080-1089 Chemistry – A European Journal, 2011, 17, 12347-12354
Chemical Communications, 2011, 47, 8775-8777 Organic and Biomolecular Chemistry, 2011, 9, 92-100
In addition, I prepared and investigated further anion templated interlocked structures, including topologically exotic handcuff catenane and Janus rotaxane species, as well as a catenane system capable of molecular motion.
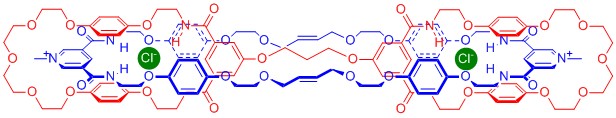
Key References:
Angewandte Chemie, International Edition, 2011, 50, 2507-2510
Chemistry – A European Journal, 2011, 17, 10541-10545
Chemistry – A European Journal, 2011, 17, 7734-7738
Luminescent Chiral Lanthanide Complexes for CPL Applications (Post-Doc, 2011-2013)
As a post-doctoral research associate, I synthesized chiral lanthanide complexes as part of a project aiming to generate luminescent probes that could be used with circularly polarised luminescence (CPL) spectroscopy to investigate chiral environments.
Installation of a single chiral centre on the triazacyclononane macrocycle of a tris-pyridyl phosphinate ligand, allowed for the generation of a single complex stereoisomer upon the addition of the Eu (III) cation, as verified by chiral HPLC. The emissive Eu (III) complexes were subsequently studied using fluorescence and CPL spectroscopies, with the mirror image CPL spectra of complex enantiomers being found to be invariant to the chiral substituent used on the ligand.

Investigations into the optimization of the photophysical properties of the complexes were also undertaken. By extending the simple pyridyl motif to a pyridyl-triazole-aryl (or pyridyl-alkynyl-aryl) chromophore, the wavelength of maximum absorption could be extended from ~270 nm to 320-350 nm, which would allow for excitation of the complexes in biological environments at wavelengths away from the most harmful region of ultraviolet radiation.
Key References:
Dalton Transactions, 2013, 42, 15610-15616
Dalton Transactions, 2014, 43, 5721-5730